The key difference between nitrate and nitrite is that nitrate contains three oxygen atoms bonded to a nitrogen atom whereas nitrite contains two oxygen atoms bonded to a nitrogen atom.
Both nitrate and nitrite are inorganic anions consisting of nitrogen and oxygen atoms. Both these anions have a -1 electrical charge. They mainly occur as the anion of salt compounds. There are some differences between nitrate and nitrite; we will be discussing those differences in this article.
What is Nitrate?
Nitrate is an inorganic anion having the chemical formula NO3–. It is a polyatomic anion that has 4 atoms; one nitrogen atom and three oxygen atoms. The anion has -1 overall charge. The molar mass of this anion is 62 g/mol. Also, this anion is derived from its conjugate acid; nitric acid or HNO3. In other words, nitrate is the conjugate base of the nitric acid.
In brief, nitrate ion has one nitrogen atom in the centre that binds with three oxygen atoms via covalent chemical bonding. When considering the chemical structure of this anion, it has three identical N-O bonds (according to the resonance structures of the anion). Hence, the geometry of the molecule is trigonal planar. Each oxygen atom carries a − 2⁄3 charge, which gives the overall charge of the anion as -1.
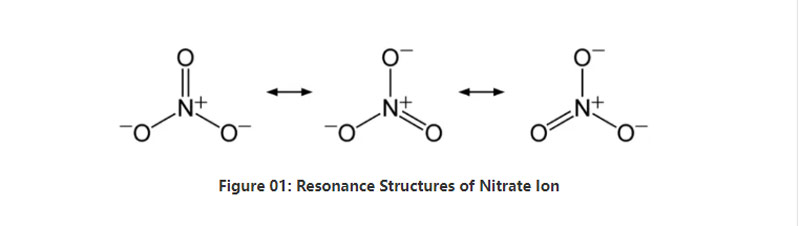
At standard pressure and temperature, almost all the salt compounds containing this anion dissolves in water. We can find naturally occurring nitrate salts on earth as deposits; nitratine deposits. It mainly contains sodium nitrate. Moreover, nitrifying bacteria can produce nitrate ion. One of the major uses of the nitrate salts is in the production of fertilizers. Furthermore, it is useful as an oxidizing agent in explosives.
What is Nitrite?
Nitrite is an inorganic salt having the chemical formula NO2–. This anion is a symmetric anion, and it has one nitrogen atom bonded to two oxygen atoms with two identical N-O covalent chemical bonds. Hence, the nitrogen atom is in the centre of the molecule. The anion has -1 overall charge.
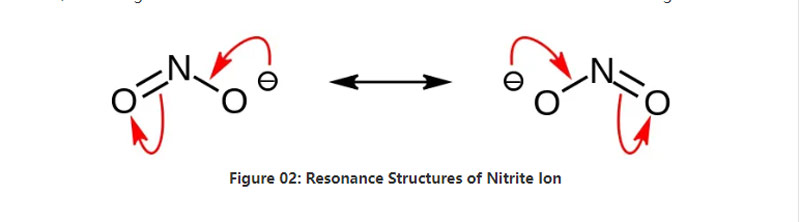
The molar mass of the anion is 46.01 g/mol. Also, this anion is derived from the nitrous acid or HNO2. Hence, it is the conjugate base of the nitrous acid. Therefore, we can produce nitrite salts industrially via passing nitrous fumes into aqueous sodium hydroxide solution. Moreover, this produces sodium nitrite which we can purify via recrystallization. Furthermore, nitrite salts such as sodium nitrite are useful in food preservation because it can prevent food from microbial growth.
What is the Difference Between Nitrate and Nitrite?
Nitrate is an inorganic anion having the chemical formula NO3– whereas Nitrite is an inorganic salt having the chemical formula NO2–. Therefore, the primary difference between nitrate and nitrite lies upon the chemical composition of the two anions. That is; the key difference between nitrate and nitrite is that nitrate contains three oxygen atoms bonded to a nitrogen atom whereas nitrite contains two oxygen atoms bonded to a nitrogen atom. Moreover, nitrate ion is derived from its conjugate acid; the nitric acid, while the nitrite ion is derived from nitrous acid. As another important difference between nitrate and nitrite ions, we can say that nitrate is an oxidizing agent because it can undergo the only reduction whereas nitrite can act as both oxidizing and reducing agent.
Post time: May-16-2022





